Photinide A
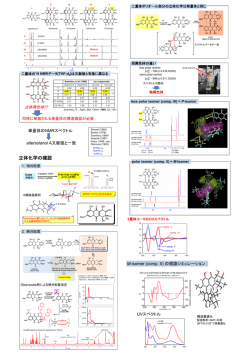
Discosia sp.が生産する benzofuranone誘導体の構造 Discosia sp. 培養液水層 培養液酢酸エチル抽出物 HPLC分析 A PDA TFAを添加した系で分離 容易に変化してしまう。 B PDAスペクトル4個を重ね書き X Y Merck LiChrospher® RP-18e (4.0×25 mm) Eluent: MeOH:H2O=30:70 Detected by UV at 290 nm ESIMS:いずれもC16H17O6 1H NMR spectra (500 MHz, acetone-d6) それぞれ類似した構造 Y X B A HMBC spectra (fr-X, in acetone-d6) 部分構造 (Fr-A) 126.45 146.13 NOE OH H LH 7.59 H 6.28 56.76 K H I G 4.55 6.77 126.45 146.13 104.54 H 37.17 A H3C NOE 6.61 O NOE H3C 56.38 3.99 F O H D 176.40 158.72 36.76 H 2.82 2.67 111.53 JH O 17.22 1.26 105.16 176.40 80.16 NOE 166.22 139.36 H 4.65 O H 2.32 C E 推定構造 HO NOE O H OMe O HO O NOE O H H CH3 CH3 OMe O Fr-A, B J. Nat. Prod., 2009, 72, 943のデータと一致 O O NOE H H O Fr-X, Y NOE 本来は非遮蔽 5.29 ppm O 間違い 6.19 ppm Photinide A 遮蔽(低周波シフト) Photinide B 非遮蔽(高周波シフト) 文献の帰属は間違っている!? 理論的化学シフトの計算 1. 配座解析(AM1法) 2. 構造再最適化(DFT B3LYP 6-311G*) 3. 量子化学に基づく化学シフト計算 Photinide A Photinide B 5.29 ppm 6.19 ppm Photinide A, Bの構造は訂正すべき Photinide B (fr-B) Photinide A (fr-A) O OMe O O OMe H O CH3 O O H O CH3 O HO HO compound X compound A Photinide A 相対立体化学 OMe O OMe HO O HO CH3 O CH3 O H O compound B Photinide B H O O compound Y O Photinide A:ERR配置 compound-X:ERS配置 CD スペクトル 実験データ (CH3CN) Photinide A (compound A) R, S R compound X 200 300 400 500 理論スペクトル(DFT/B3LYP/6-31G*) ) (E,S,S)-isomer (E,R,S)-isomer 同一、または鏡像のCDを 与える?? 200 250 300 350 400 450 ESS(calcd) ERS(calcd) 500 OMe O HO 1) O3, then H2O O O H(S) H3C OBn O O (S) H O 2) BnOH, p-TsOH H3C O (S) (S) NaphO 1) LiAlH4 ONaph 2) Naph-Cl/Py (S) ONaph positive Cotton (S) 1) LiAlH4 2) Naph-Cl/Py COOMe BzO 1) p-TsOH/MeOH D-mannnitol O (S) (S) 250 200 natural 150 synthetic 2S,3S 100 50 0 -50 -100 -150 -200 200 250 300 350 O (S) 2) separation 3) BzCl/Py O H 400 O O OMe O OMe H O (S) (E) O (S) HO compound A Photinide A O O compound X OMe HO (Z) (R) CH3 O HO OMe H O (S) (E) CH3 O O O CH3 (S) (S) H O HO (Z) O CH3 (R) (S) H O O compound B Photinide B O compound Y Photinide B (fr-B) Photinide A (fr-A) 文献データ O (R) (R) 間違い (R) (R) OMe O O HO OMe H O O (S) (S) (S) O O H(S) H3C O CH3 O HO
© Copyright 2026